Products
Clinical Diagnostic Tests
Oncoveda has created a product development program using guidelines from NIH and FDA with the goal to efficiently bring sensitive and specific cancer testing into the clinical diagnostic lab. Our product development involves investment in basic research to identify and validate potential biomarkers (Biomarker Identification), as well as in applied research to translate these findings into useable diagnostic tools (Assay Development). We then test our diagnostic assays in a prospective clinical trial (Clinical Validation) before final transfer to our clinical diagnostic lab (Clinical Transfer) or other partners for commercialization.
Cancer Diagnostic Development:
Click On Graph To Enlarge
Small Compound Therapeutics
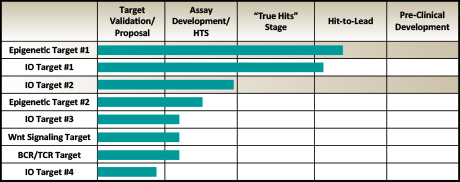
Oncoveda is integral to the Genesis Biotechnology Group drug discovery program, which is designed to take potential therapeutic compounds into pre-clinical testing. Our contribution to the drug discovery program is research to identify and validate suitable oncology drug targets (Drug Target Evaluation). Venenum Biodesign, L.L.C. carries the program through high-throughput screening against their proprietary 5.5 million ECLiPS compound collection, hit-to-lead optimization, and pre-clinical validation.
Cancer Drug Discovery:
Click On Graph To Enlarge
Latest News
Updated Website! - The Oncoveda website has been fully updated! Please take a moment to learn about our current programs and scientific members!
Projects for Lab Rotations/Internships within Oncoveda - Please access the Research Page (link above) to learn about the lab rotation/internship projects that are currently available in Oncoveda.
Article Accepted into Tumor Biology - Our article further describing the role of PME-1 in endometrial cancer tumor progression titled "Inhibition of Protein Methylesterase 1 decreased cancerous phenotypes in endometrial adenocarcinoma cell lines and xenograft tumor models" has been accepted for publication in Tumor Biology.